A Carboxyl Group Contains Which of the Following Atoms
Formula of Carboxyl group. Highlight all the atoms of the four functional groups but do not highlight any bonds.
What Are Carboxylic Acids Quora
All amino acids have a central carbon atom surrounded by a hydrogen atom a carboxyl group COOH an amino group NH2 and an R-group.

. All amino acids have a central carbon atom surrounded by a hydrogen atom a carboxyl group COOH an amino group NH2 and an R-group. Carboxyl functional group the carboxyl group COOH contains two O atoms that pull electrons away from the H atom so this group loses a proton and is acidic. A fatty acids has a carboxyl group attached to an R group.
B form between fatty acids. Carboxyl groups have the formula -C OOH usually written as -COOH or CO 2 H. Describes the alpha-helices and beta-sheets that are formed by hydrogen bonding between backbone atoms located near each other in the polypeptide chain.
The amino acid contains only two carbon atoms. Palmitic acids has 16 carbons atoms including carboxyl carbon Archidonic acid has 20 carbon atoms including the carboxyl carbon. Expert Answer Generally a fatty acid consist of a straight chain of an even number of carbon atoms with hydrogen atoms along the length of the chain and at one end of the chain and a carboxyl group -COOH at the other end.
Both Assertion and Reason are incorrect. Carboxyl group of AA1 and amino group of AA2. A A B B C C D C and E E none of the structures Answer.
H2N-CHR-COOH All amino acids differ from one another in terms of -R group. Nucleotide monomers make up nucleic acids. The amino group is attached to the carbon atom of the carboxyl group.
It is that carboxyl g. This second oxygen is also bonded to a hydrogen atom. When you click on each atom it will change color.
Likewise what is in a carboxyl group. See answer 1 Carboxylic acids ketones aldehydes and esters all contain a carbonyl group. The molecule shown here contains four functional groups.
A carbonyl group between two carbon atoms. B the chemical properties of their R groups. 31 Peptide bonds D A are used to form amino acids.
The Carboxyl group is attached to some other elements. A Carboxyl Group is a functional organic compound that comprises a double-bonded carbon atom linked to an oxygen group and a hydroxyl group through a single bond. Carboxyl functional group the carboxyl group COOH contains two O atoms that pull electrons away from the H atom so this group loses a proton and is acidic.
First week only 499. KnowledgeComprehension 40 In which. Oxygen atom bonded to two carbon atoms d.
They include acetic acid and amino acid. Here are the structures of some common carboxylic acids. Start your trial now.
The carboxyl group occurs on the end or side of a molecule. In chemistry the carboxyl group is an organic functional group consisting of. The carboxyl group COOH contains two oxygen atoms that tend to pull electrons away from the hydrogen atom so this group tends to lose a proton and is acidic Which monomers make up RNA.
The general formula of amino acids is given as. The carboxyl group COOH is present in all carboxylic acids. Each amino acid has the same fundamental structure which consists of a central carbon atom also known as the alpha α carbon bonded to an amino group NH2 a carboxyl group COOH and to a hydrogen atom.
The general formula of amino acids is given as. Ethers do NOT contain a carbonyl group. 39 which of the structures contains a carboxyl.
Identify the class of compounds that contains each of the following functional groups. The amino group and the carboxyl group are directly bonded to the same carbon atom. Oxalic acid contains two COOH groups and citric acid contains three.
Carboxyl and amino An amino acid may be defined as an organic compound which contains amine -NH2 group a carboxylic -COOH group and alkyl -R group. The Carboxyl group can be presented as RCOOH. Carboxyl groups are often seen in organic elements such as Carboxylic acids.
The group consists of a carbon atom that forms two chemical bonds to one oxygen atom and one chemical bond to a second oxygen atom. The R group could be a methyl CH 3 or ethyl C 2 H 5 or higher number of CH 2 group 1 carbon to 19 carbons. A nitrogen atom attached to one or more carbon atoms b.
Nitronium ion consists of one nitrogen atom and two oxygen atoms. An organic compound consisting of a carboxyl group is termed as a carboxylic acid. View the full answer.
The compound formed gives many properties. Which of the following statements concerning the structure of α-amino acids is correct. A carboxyl group COOH is a functional group consisting of a carbonyl group CO with a hydroxyl group O-H attached to the same carbon atom.
Jul 25 2015 The carboxyl group COOH is present in all carboxylic acids. Therefore Amino and carboxyl groups are always found in amino acids. Number of lone pairs prese.
Oxalic acid contains two COOH groups and citric acid contains three. Carboxylic acids contain the carboxyl group which is a carbonyl group attached to a hydroxyl group. The amino acid contains only one carbon atom.
Solution for Which of the following compounds contains a carboxylic acid group. 39 Which of the structures contain s a carboxyl functional group. Ester contains the carboxyl group between carbon atoms Amine the functional group is a nitrogen atom Amide the hydroxyl group of a carboxylic acid is replaced by a nitrogen group Recommended textbook explanations Organic Chemistry.
The molecule shown here contains four functional groups OH SH NH2 and OPO2 3 attached to a carbon chain that is six carbon atoms long. The Carboxyl group consists of carbon having Bonded with oxygen and hydroxyl group. D the chemical properties of their amino and carboxyl groups.
Each amino acid has the same fundamental structure which consists of a central carbon atom also known as the alpha α carbon bonded to an amino group NH2 a carboxyl group COOH and to a hydrogen atom. C are formed by a hydrolysis reaction. Carboxyl groups are commonly found in amino acids fatty acids and other biomolecules.
C the type of bond between the R group and the rest of the amino acid molecule. 1 Answer Ernest Z. Which of the following compounds contains a carboxylic acid group.
What Is A Molecule That Includes Hydroxyl Carboxyl And Amide Groups Quora
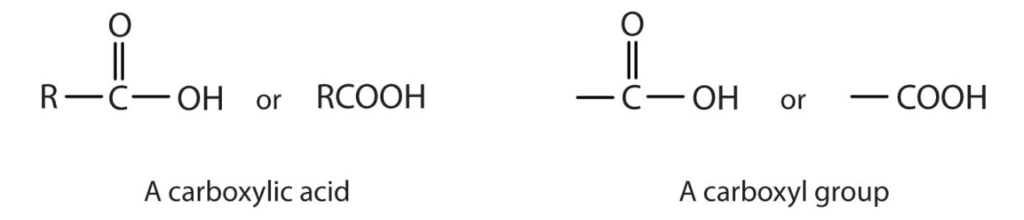
Comments
Post a Comment